Areas of investigation/research focus
My group is interested in elucidating pathways regulating immune cell function in ageing and neurodegenerative diseases, with a particular focus on myeloid cells, the components of the innate immune system.
In particular, we are interested in understanding the changes that characterize immune cells, such as microglia and neutrophils, with age and in Alzheimer’s disease, with the scope of identifying new pathways and molecular targets that can lead to therapeutic interventions.
Signaling pathways regulating inflammation in aged microglia
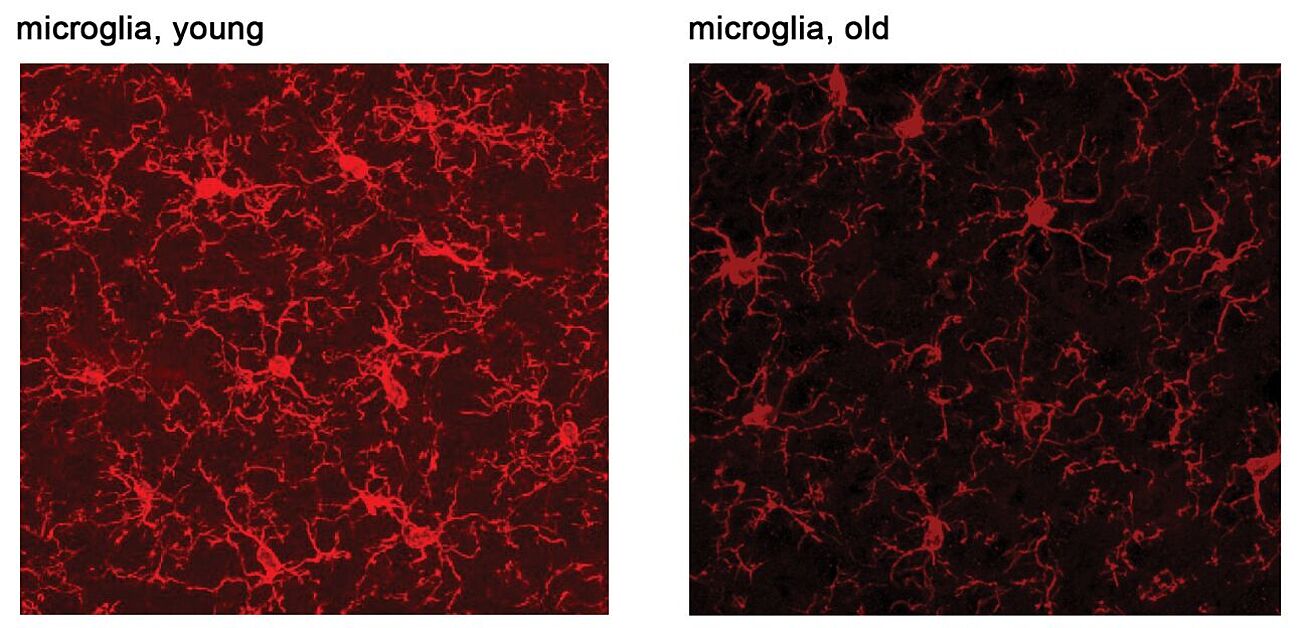
Microglia are the immune cells of the brain, they play important roles in neuronal cell homeostasis but can contribute to disease pathology in neurodegenerative diseases, through the production of inflammatory factors. It is increasingly recognized that microglia become progressively impaired with age (Figure 1), however, it remains to be further clarified which microglia functions and at which temporal stage confer resilience or vulnerability to biological insults related to aging, as well as their relationship to neurodegeneration (reviewed in Antignano et al., CMLS, 2023). Nonetheless, excessive inflammatory responses in the form of cytokine production are well-established deleterious events that can be detrimental for neuronal health. While studying alterations in signaling pathways that regulate inflammatory responses in aged microglia, we discovered an upregulation of the mTORC1 pathway, especially its regulation of protein synthesis. Indeed, during an inflammatory response, aged microglia synthesize greater amounts of inflammatory mediators, such as cytokines and immune receptors (Keane*, Antignano* et al., JCI, 2021; Antignano et al., STAR Protocols, 2023). Genetic inhibition of mTORC1 signaling led to decreased inflammation because of reduced protein mediators, despite their mRNA expression levels being higher. Surprisingly, mTORC1 inhibition appeared to have a rather specific effect on translation of the inflammatory response, however, it plays a role on mRNA translation overall by regulating different steps of ribosomal biogenesis, translation initiation and elongation. In order to fully understand the impact of interfering with mTORC1 in microglia in aging, we are now exploring the specificity of mTORC1 regulation over the microglia translatome, as well as exploring pathways that lead to mTORC1 upregulation (through the EU-funded DN-MSCA Eternity) as well as, more generally, alterations in translation regulation in aged microglia.
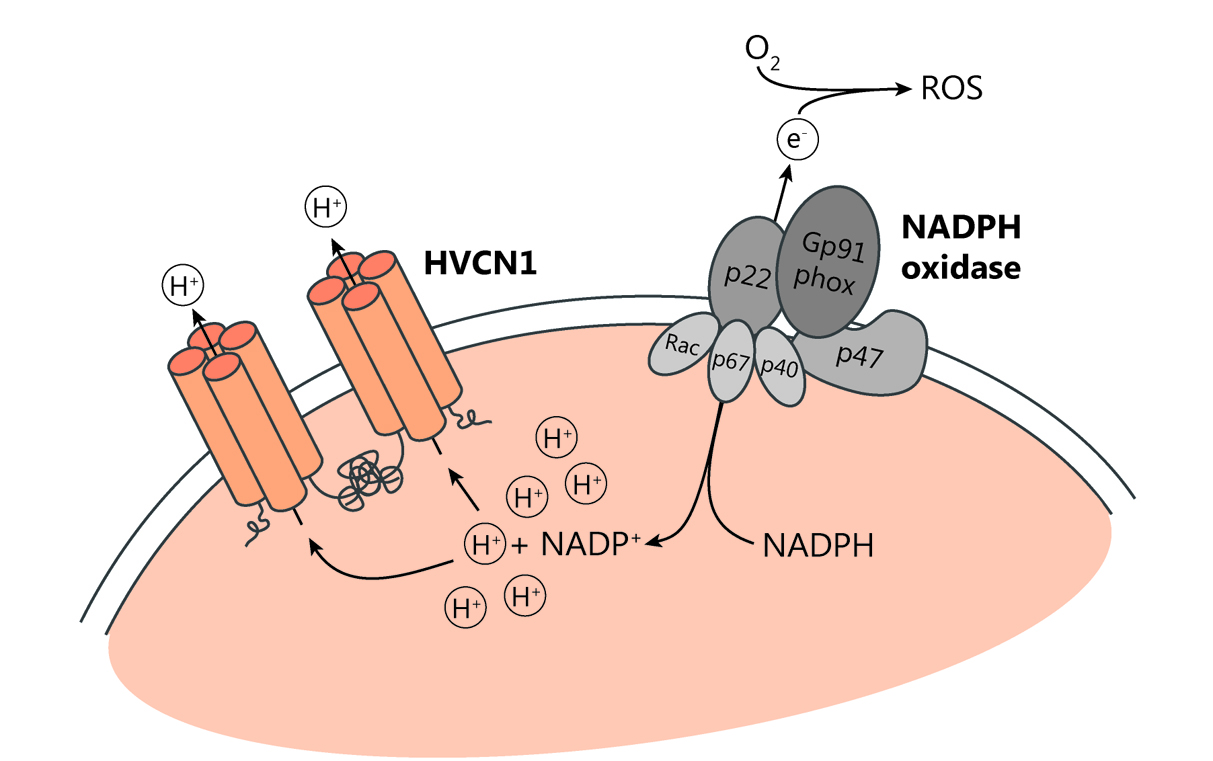
Studying proton channels to identify potential new target in Alzheimer’s disease
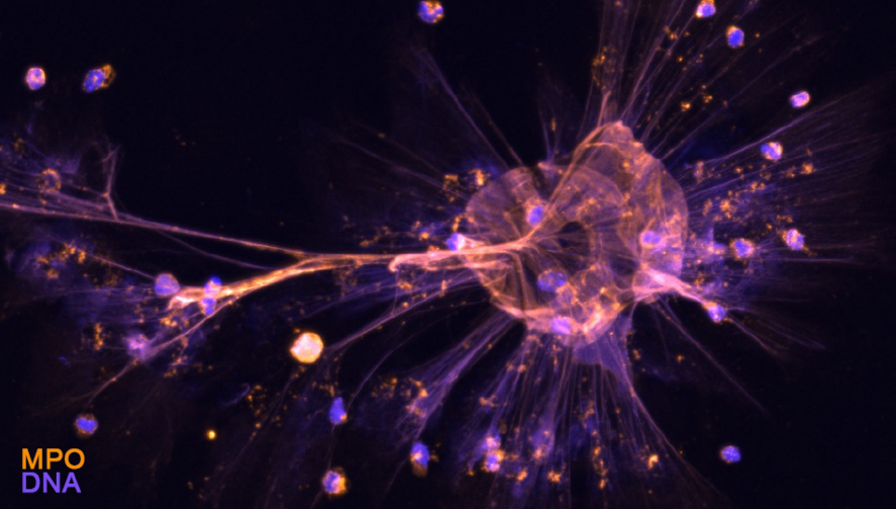
Our lab has a long-standing interest in proton channels, which we found to regulate B and T cell responses, as well as neutrophil functions (Coe et al., JCI Insight, 2022; Hondares et al., PNAS, 2014; Capasso et al., Nat Immunol, 2010; Morgan et al., PNAS, 2009). There is only one mammalian voltage-gated proton channel, HVCN1, whose function is to lessen intracellular acidification and membrane depolarization, letting protons out of the cell following their chemical gradient. In doing so, it sustains the production of reactive oxygen species (ROS) by the enzyme NADPH oxidase (Figure 2). In myeloid cells, HVCN1 also sustains NADPH oxidase activity, however, its role in cells implicated in the pathogenesis of Alzheimer’s disease, such as microglia and neutrophils (Figure 3), remains to be elucidated. Proton channels appear to regulate cell functions not directly linked to the NADPH oxidase-ROS production; we are combining in vivo and in vitro studies to elucidate the impact of HVCN1 loss on the function of these cells, at steady state and in the context of Alzheimer’s disease.