Research areas/focus
Proteins are the ‘work horses’ of cells and enable almost all cellular functions, from genome organization over signaling cascades up to the mechanical support of cellular structures and transport of molecules. Neurons are especially polarized non-dividing cells with extreme extensions and diverse microenvironments, which asks for robustly regulated and highly specialized protein actions and functions in different compartments and situations. In neurodegenerative diseases and during aging, protein actions can change, and loss of function, misfunctions, and aggregation of proteins are commonly observed.
In our group we use a wide spectrum of experimental methodologies and techniques – from biophysical and biochemical techniques, to cell culture and animal models, and to postmortem human tissue - to discover the actions that proteins take during their normal function and during misfunction in the diseased brain. Thereby we currently focus on (but are not exclusive about it) the intrinsically unfolded neuronal microtubule-associated tau protein, which aggregates in multiple different neurodegenerative diseases. But tau also seems to harbor a variety of other functions in the cell that yet need to be explored. Identifying such unusual functions of tau (and other proteins) in the brain provides the opportunity to explain toxicity effects and enables the development of novel therapeutic approaches.
The following research areas are currently addressed in our group:
1. (Patho)Physiological activators of Tau in the brain
The microtubule associated protein Tau (MAPT) is abundant in neurons of the central nervous system, where it is thought to be localized mostly to axons in healthy conditions. Different neurological conditions “activate” Tau leading to its elevated phosphorylation and accumulation in the somato-dendritic compartment. The reason for this “re-sorting” is not known. In disease conditions, such as in Alzheimer’s disease and primary tauopathies, the aggregation of highly phosphorylated Tau in neuronal cell bodies represents a pathological hallmark that correlates with neuronal death and cognitive decline. Phosphorylation and somato-dendritic resorting of Tau are therefore often interpreted as pro-pathological events. In our group, we investigate which physiological conditions can trigger Tau activation and try to answer the How and What-for of it. To understand the actions of Tau and what triggers them, we investigate, for example, the conditional interactomics of Tau and use cellular and animal models of the autoimmune-antibody induced tauopathy IgLON5 disease, which allows us to study the mechanisms leading to Tau activation in the brain from a unique angle.
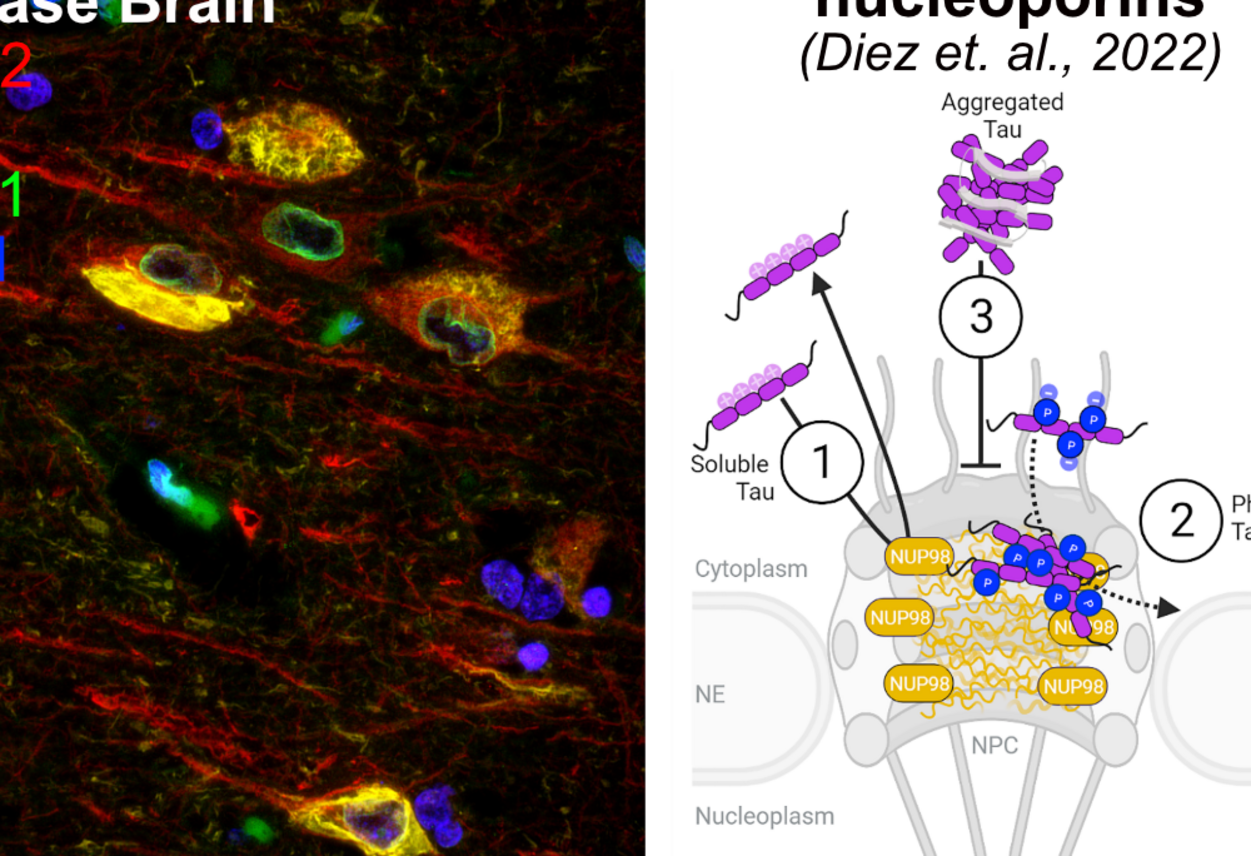
2. Novel and unusual functions of the microtubule associated protein Tau
Tau has been reported to be involved in a number of cellular processes that are presumably independent of its prominent binding to axonal microtubule. We are especially interested in interactions acquired by Tau upon its “activation” (phosphorylation and somato-dendritic occurrence) since these interactions may represent functions of Tau in physiological neuronal stress response that, in the long run, could lead to pathological alterations in neuronal physiology, neurotoxity, and protein aggregation. Currently, we are focusing on the interactions of Tau with the neuronal nucleus since this essential organelle is encountered by phospho-Tau in both normal neuronal stress response and early in disease. Our studies address the interaction of Tau with nucleocytoplasmic transport effectors (e.g., nuclear pores and nuclear transport factors) as well as its consequences for neuronal chromatin organization and gene expression.
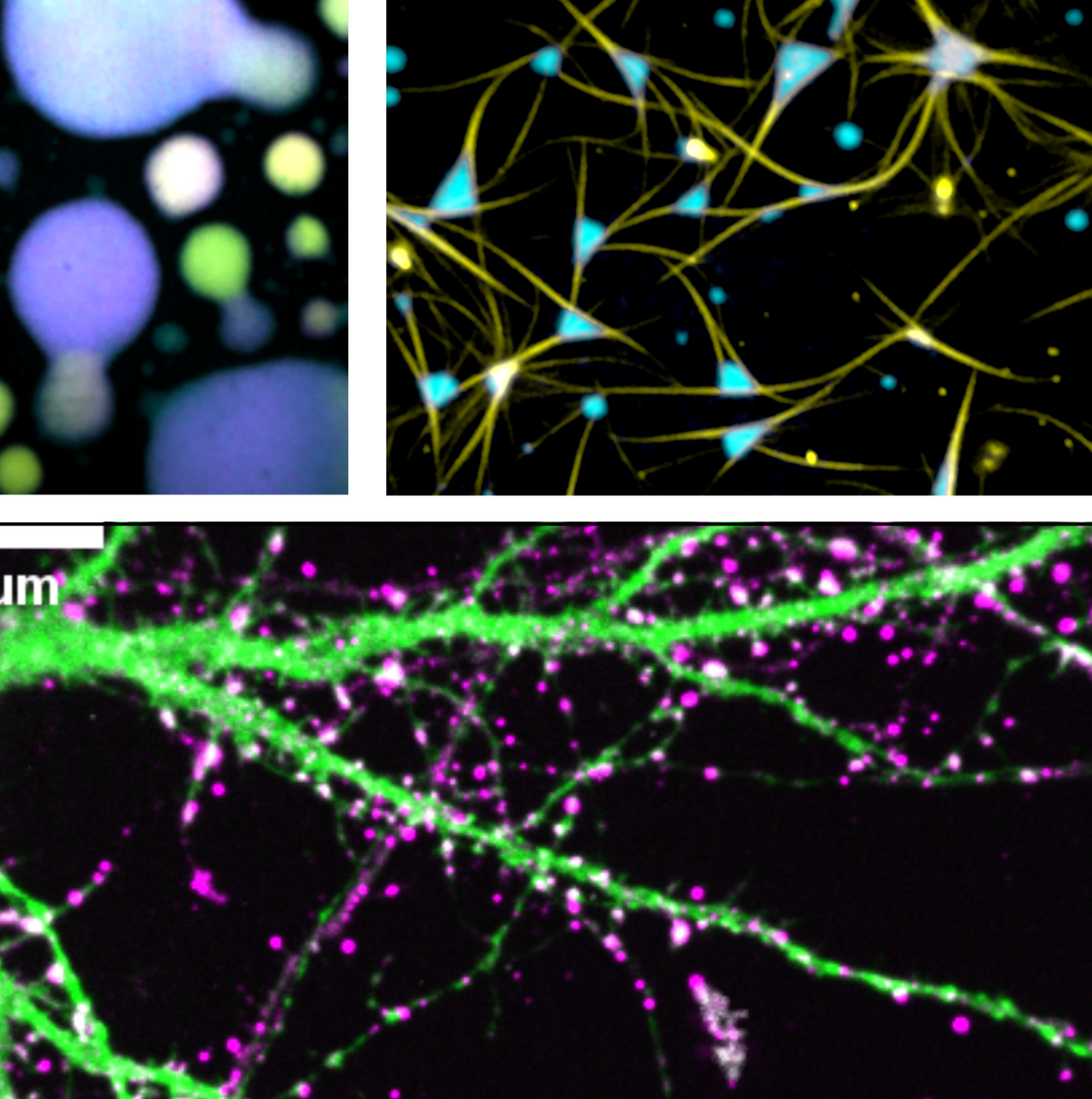
3. Condensed-phase biology of Tau
Tau has the capability to assemble into different multimeric states, including physiological dimers, small soluble oligomers, beta-structure rich fibrillar aggregates, and liquid-like condensates. We are especially interested in the characteristics and unique biological potential of condensed phases of Tau. This assembly state can not only induce pathological Tau aggregation, but also provide us with room for the re-interpretation of previous (in part controversial) findings around Tau misfunctions, and they boost the understanding of how Tau can influence different cellular processes and be kept in control for most of the brain’s life.











